ETHICS REVIEW COMMITEE
Latest Updates
About the CNU Ethics Review Committee
The CNU Ethics Review Committee (CNU ERC) was approved by the Board of Regents on December 7, 2017 through Resolution No. 117 series of 2017. Subsequently, the approval of the CNU ERC Standard Operating Procedures, Special Guidelines and Ethical considerations (Resolution No. 14 series of 2018) was given on March 8, 2018. The CNU ERC is designated to approve, monitor, and review biomedical and behavioral research involving humans and all forms of life with the aim of protecting the rights and welfare of the research subjects.
The Board shall have the following functions:
- Require all faculty members and students who will conduct research that involves human participants to submit a complete description of the proposed research using the CNU ERC Protocol Submission Overview;
- Review all researches submitted by faculty members and students that involve human participants to assure, both in advance and by periodic review, that appropriate steps are taken to protect the rights and welfare of humans
participating as subjects in a research study: - Assess the ethics of the research and its methods, to promote fully informed and
voluntary participation by prospective subjects who are themselves capable of making such choices (or, if that is not possible, informed permission given by a suitable proxy) and to maximize the safety of subjects once they are enrolled in the project; and - Approve or disapprove the implementation of the research project or withhold approval of the implementation of the study pending modifications or changes to protocol or the consent procedures.
Ethical Considerations
The CNU Ethics Review Committee (ERC) is dedicated to assisting student and faculty researchers in ensuring the ethical integrity of their research protocols. By adhering to its ethical guidelines, the ERC ensures that research conducted under its purview is ethically sound, respects the rights and dignity of participants, and maintains the highest standards of academic integrity. To uphold high ethical standards, the ERC requires all submitted research protocols to include provisions addressing the following ethical considerations:
- Voluntary Participation:
Participants should not be coerced through bribery, pressure, or blackmail into participating in research. Every participant must have the autonomy to choose to be involved in the study and must retain the right to withdraw at any time without any repercussions. - Anonymity and Confidentiality:
Anonymity involves ensuring that participants remain unidentifiable and untraceable, while confidentiality pertains to the non-disclosure of collected data to unauthorized individuals or entities. Research protocols must clearly outline the methods for collecting, storing, processing, and disposing of data to safeguard participants’ identities and privacy. - Conflict of Interest:
A conflict of interest arises when personal interests could compromise an individual’s judgment or decision-making during the research process. Researchers must explicitly declare any potential conflicts of interest to maintain transparency and integrity in their work. - Informed Consent or Assent:
Participants of legal age (18 years and older) must provide informed consent, whereas minor participants (under 18 years) must provide assent, accompanied by a child information sheet and a consent form from their parents or legal guardians. The informed consent form should include a brief description of the study, its objectives, the extent of participant involvement, potential risks and benefits, and contact information for the primary investigator. - Risks:
Researchers must outline the potential harms that participants may face if they choose to participate in the study. These risks can be physical, social, psychological, or legal. Identifying potential risks allows researchers to minimize their impact and to develop solutions and support mechanisms should these risks materialize. - Benefits:
Participants may incur personal expenses or face inconveniences during data collection. Therefore, they should be justly compensated when applicable. For instance, traveling to and from an interview location may require reimbursement for transportation and food expenses. Additionally, if the data collection impedes on the productive economic hours of the participants, the researchers must give compensation to the participants equivalent to the number of hours they have allotted for the study.
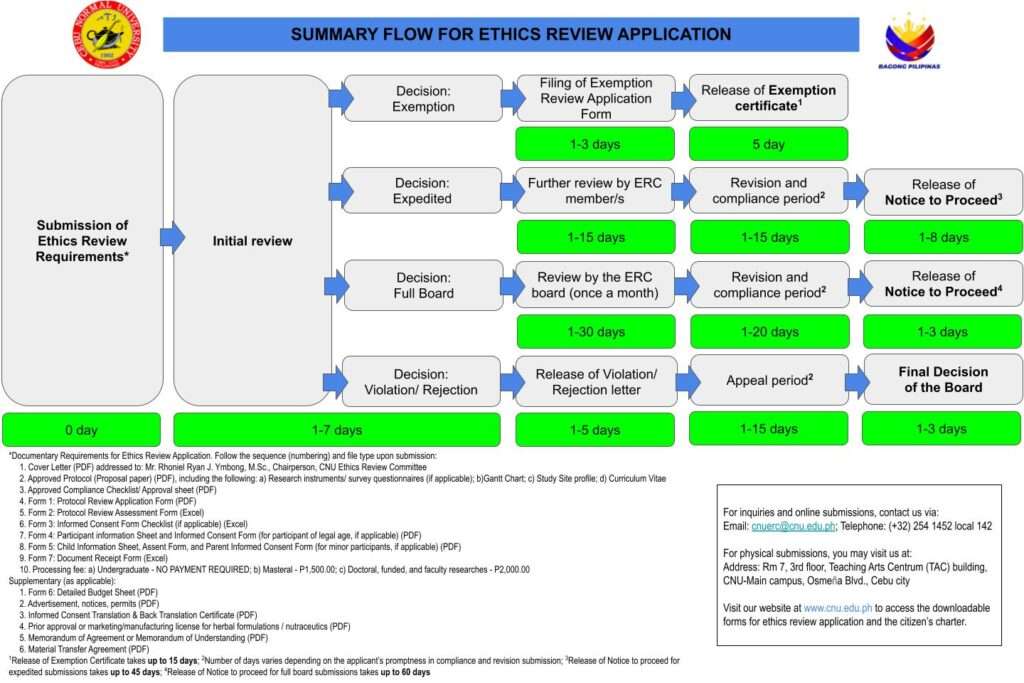
List of documentary requirements for Ethics Review Application
All of the following documents must be submitted to the ERC office for protocol ethics review. Make sure that your submission is complete. All forms (except for the cover letter, approved manuscript, and completed compliance form) are provided by the ERC office.
- Cover letter (addressed to the ERC Chair)
- Approved Manuscript (w/ CV, Gantt Chart, Research Instrument)
- Completed Compliance form
- Form 1: Protocol review application form
- Form 2: Protocol review assessment form
- Form 3: Informed consent form checklist
- Form 4: Participant information sheet and informed consent
- Form 5: Child information sheet and assent form
- Form 6: Detailed budget sheet
- Form 7: Document receipt form
Downloadable Forms
- FORM 1 Protocol review application form
- FORM 2 Protocol review assessment form
- FORM 3 Informed consent form checklist
- FORM 4 Participant information sheet and informed consent
- FORM 5 Child information sheet and assent form
- FORM 6 Detailed budget sheet
- FORM 7 Document receipt form
- FORM 8 Exemption from Review Application
- FORM 9 Reportable Negative Event Form (RNEF)
- FORM 10 Premature, Suspension, Discontinuation Report Form
- FORM 11 Study Completion/ Final Report Form
- FORM 12 Assessment of resubmitted protocol
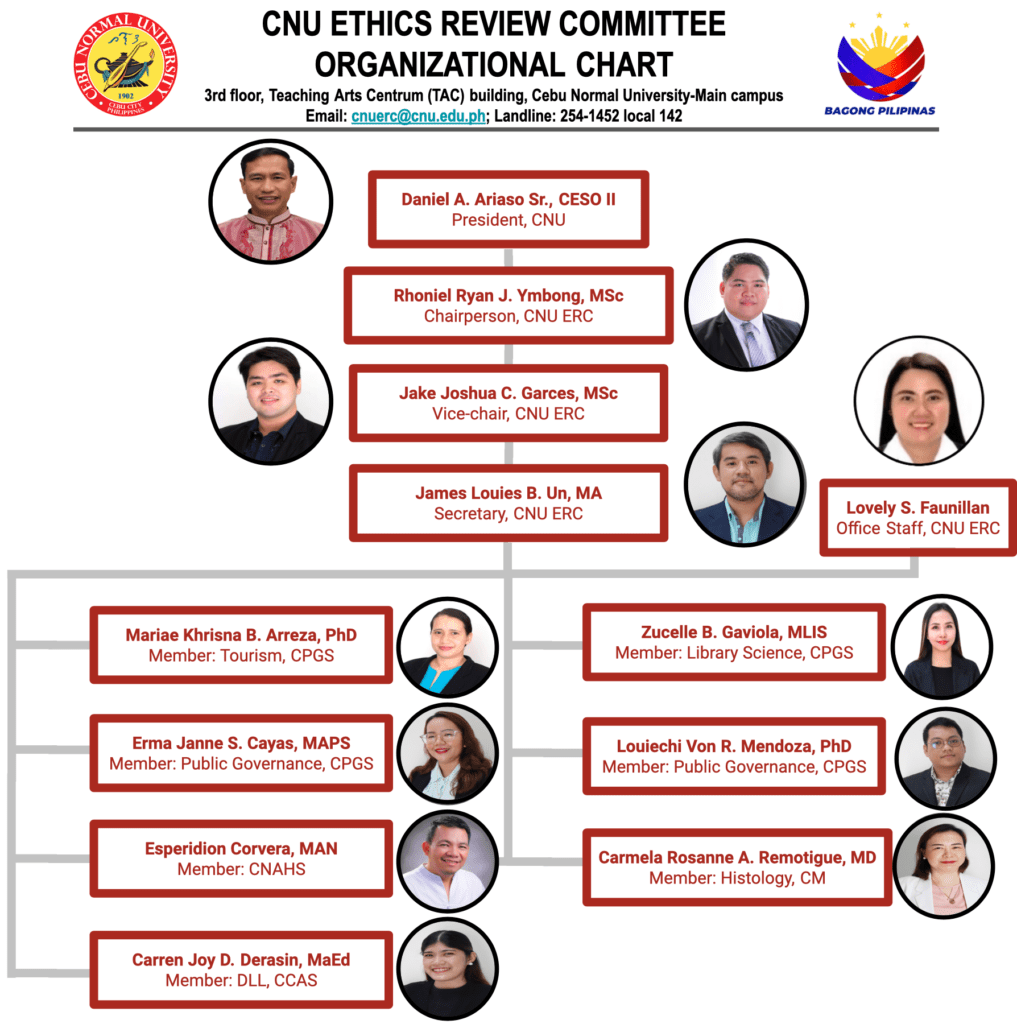
Organizational Chart
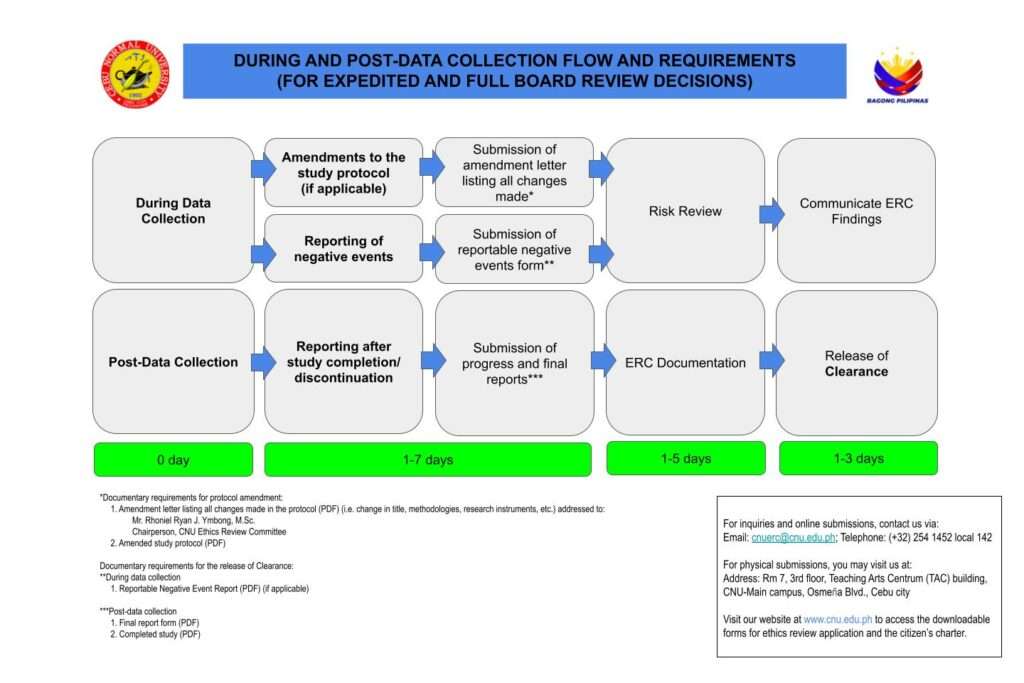
Ethics Review Process
Contact Information
Rhoniel Ryan J. Ymbong, M.Sc.
Chair, Ethics Review Committee
ymbong@cnu.edu.ph
For more inquiries and online submissions, contact us via:
Email: cnuerc@cnu.edu.ph; Telephone: (+32) 254 1452 local 142
For physical submissions, you may visit us at:
Address: Rm 7, 3rd floor, Teaching Arts Centrum (TAC) building, CNU-Main campus, Osmeña Blvd., Cebu city
Visit our website at www.cnu.edu.ph to access the downloadable forms for ethics review application and the citizen’s charter.